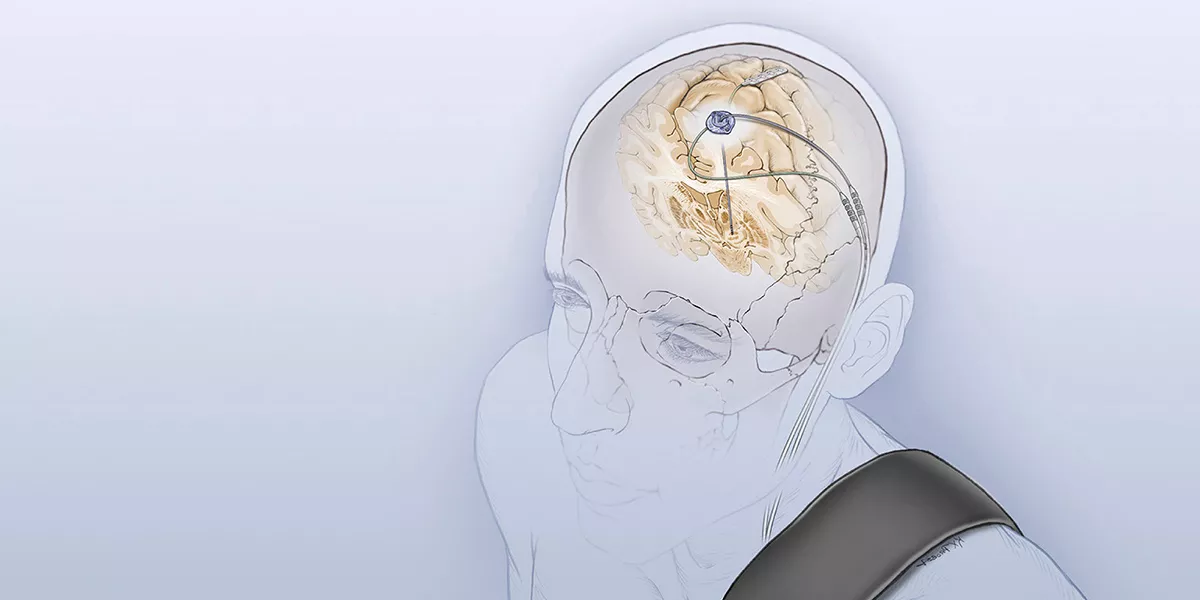
UC San Francisco leads a new consortium funded by a $4.4 million NIH grant from the NIH BRAIN Initiative to develop open-source technology platforms for a new generation of neurostimulation devices that not only provide stimulation to the brain but also sense, record, and stream brain activity.
Dubbed the OpenMind Consortium, the leadership group also includes investigators at Brown University, Mayo Clinic, and the University of Oxford. “These four groups have been the earliest investigators to work on these advanced new neurostimulation devices and together bring the highest levels of expertise in this field,” said UCSF neurosurgeon and principal investigator for the grant, Philip Starr, MD, PhD.
“As we move toward modulating brain circuitry in real-time, the increasing complexity of the technology requires collaboration among neuroscientists, computer scientists, and engineers to develop safe and effective interventions that also adhere to neuroethical principles,” said John Ngai, PhD, director of the NIH BRAIN Initiative. “Moreover, one of the major goals of the BRAIN Initiative is to make new technology widely available to help people affected by neurologic and neuropsychiatric disorders.”
Traditional stimulation devices to treat neurological disorders like Parkinson’s disease provide constant electrical stimulation to the brain in an effort to disrupt aberrant neural circuits that lead to symptoms. The new generation of devices provide stimulation, but for the first time also have the ability to record brain signals and stream high volumes of that data outside the lab as patients go about their normal activities in the real world.
By correlating these data with patients’ symptoms, investigators hope to better understand the neural circuits underlying brain disorders - ranging from epilepsy and movement disorders to severe psychiatric disorders - and develop personalized, intelligent therapies to normalize circuits that have gone awry. This would not only avoid side effects associated with constant stimulation but allow for a therapy tailored to an individual patient.
While representing enormous potential for better treatment of many poorly understood brain disorders the new devices are also technologically complex, imposing significant barriers to use by researchers.
“Part of the reason the device is so flexible and tailorable is that the investigators write the software that controls it, which is something only device companies have been doing in the past,” said Starr. “We also are therefore responsible for documenting that the device works according to FDA regulations.”
Writing custom software for the device is not only costly and time-consuming but requires specific knowledge regarding security and quality controls. All of these are necessary for safety, but also pose hurdles to technological development in academic settings and led to a growing awareness at the NIH of the need for a consortium of experts to help disseminate enabling technology resources. One of the goals for the Open Mind Consortium is to leverage the best tools and ensure that they are made available as quickly as possible. These include best-practice guidance and generalizable software applications that can be upgraded as the current devices phase out and new versions come to market.
“Our group at UCSF and the other groups in the consortium have already overcome these developmental hurdles, so our goal will be to not only disseminate tools and technologies that can be used by the wider research community, but also work with the NIH and manufacturers to develop standards on software architecture, security features, data sharing, and neuroethics for others applying for grants that use these new devices,” said Heather Dawes, PhD, OpenMind administrative director.
A Steering Committee that includes neuroethicists and patient advocates will also advise on the development of these standards as they relate to patient consent and control of the device and streaming.
By creating a common technical and regulatory infrastructure for the devices, Open Mind ultimately aims to create a more streamlined regulatory pathway for FDA approval of investigational protocols.
The OpenMind Consortium is funded by NIH grant number 1U24NS113637-01A1. Principal Investigators include:
Philip Starr, MD, PhD, Dolores Cakebread Professor of Neurological Surgery, Dept. of Neurological Surgery, UCSF
David Borton, PhD, Assistant Professor of Engineering, School of Engineering and Carney Institute for Brain Science, Brown University
Gregory Worrell, MD, PhD, Professor, Dept. of Neurology, and Division Chair, Clinical Neurophysiology, Mayo Clinic
Tim Denison, PhD, Royal Academy of Engineering Chair in Emerging Technologies, Institute of Biomedical Engineering, University of Oxford