Astrocytes – a unique population of star-shaped brain cells – are selectively vulnerable to the perturbations that underlie the neurological symptoms in Down syndrome, Alexander disease, and SARS-COV-2 virus infection. But, very little is known about how astrocytes form in the developing brain.
Tomasz Nowakowski, PhD, an assistant professor in the departments of neurological surgery and anatomy, and his basic science research team seek to address this question with new funding from a National Institutes of Health (NIH) Research Project grant. Nowakowski, who is also a faculty member in the Eli and Broad Center for Regeneration Medicine and Stem Cell Research, hopes that his lab’s research on the developmental origins of these cells could reveal new approaches to study astrocyte development in cell lines and brain organoids. Their findings may also help researchers develop novel tools to target astrocytes in neurological diseases and psychiatric disorders.

Astrocytes and their developmental origins have been primarily studied in mice. However, the human brain is different and contains many more subtypes of astrocytes.
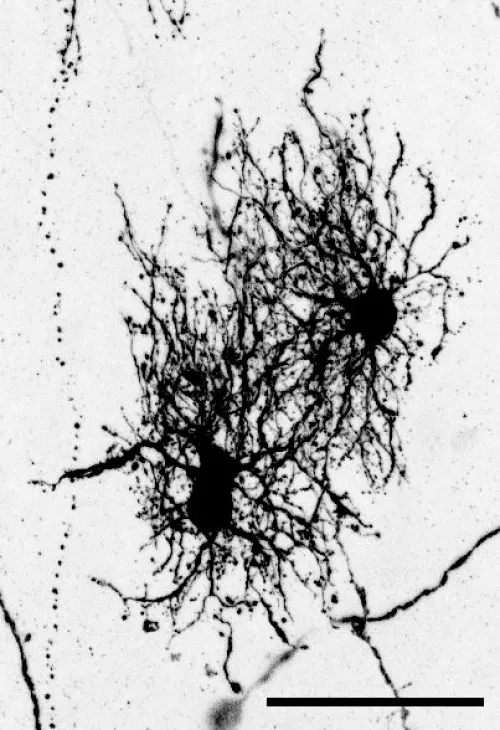
In a study published earlier this year in Science, Denise Allen, a recent graduate from Nowakowski’s lab, used a virus to label neural stem cells with a fluorescent protein to see the morphology of astrocytes they developed.
Though a collaboration with Vikaas Sohal, MD, PhD, an associate professor in the Department of Psychiatry, the scientists also sequenced all the genes from single astrocytes, identifying novel markers specific to these cells.
Unlike neurons, astrocytes are quite similar to one another when you compare their gene expression profiles. But by connecting the information about their morphology, their molecular identity, their developmental origin, and their position, Allen established a comprehensive, unbiased scheme for classifying astrocytes. “There is a lot more heterogeneity that that had been not appreciated until now,” Nowakowski said.
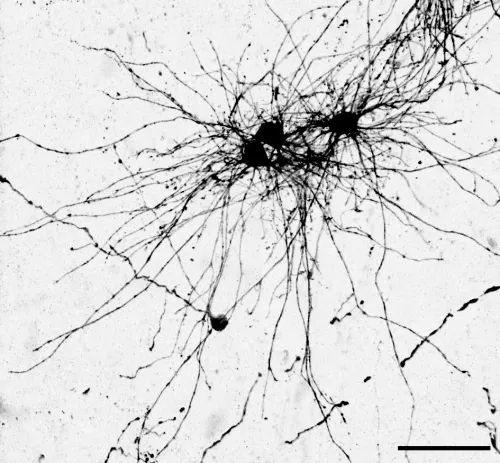
Allen also found that two subtypes often cited in the scientific literature – white matter and grey matter astrocytes – come from different stem cell niches. Rather than one unique stem cell niche that goes through a sequence, Nowakowski says the two niches can act simultaneously to make neurons, astrocytes, and oligodendrocytes.
“It completely changes the way we think about neural stem cells,” he said.
Nowakowski is now interested in further delineating the dynamics of when the different types of astrocytes are born. He wants to resolve whether these various cells all come from the same progenitor cells in a similar manner to the hematopoietic stem cells that produce new blood cells, which can adapt depending on what the body needs. Alternatively, certain neural stem cells may be set aside to generate the different brain cell types, he says.
Scientists are still debating how a brain cell’s developmental origin may influence its function, but Nowakowski argues that certain neural progenitor cells seem to selectively generate specific cell types.
“I'm fascinated by the question of why there are so many cell types in the brain,” Nowakowski said. “This is a fundamental question in neuroscience.”
Several studies also indicate that disruptions to neural development – either through DNA mutations or viral infections – can contribute to psychiatric disorders by changing the balance of different cell types that are being produced.
Creating a “roadmap” of progenitor cells during human brain development, Nowakowski says, will not only enable scientists to ask more specific questions about which cell types may be affected and how, but will also help develop new strategies to derive these cells from pluripotent stem cells for future applications in regenerative medicine.
