About a third of people with epilepsy have seizures that do not respond to the currently available medications.
This figure, according to Scott C. Baraban, PhD, the William K. Bowes Jr. Endowed Chair in Neuroscience Research at UC San Francisco, unfortunately has not budged over the last 80 years. With a $3.4 million grant from the National Institutes of Health (NIH), Baraban and his team continue a research program initially funded in 2010 to bring this therapy one step closer to patients with intractable epilepsies.
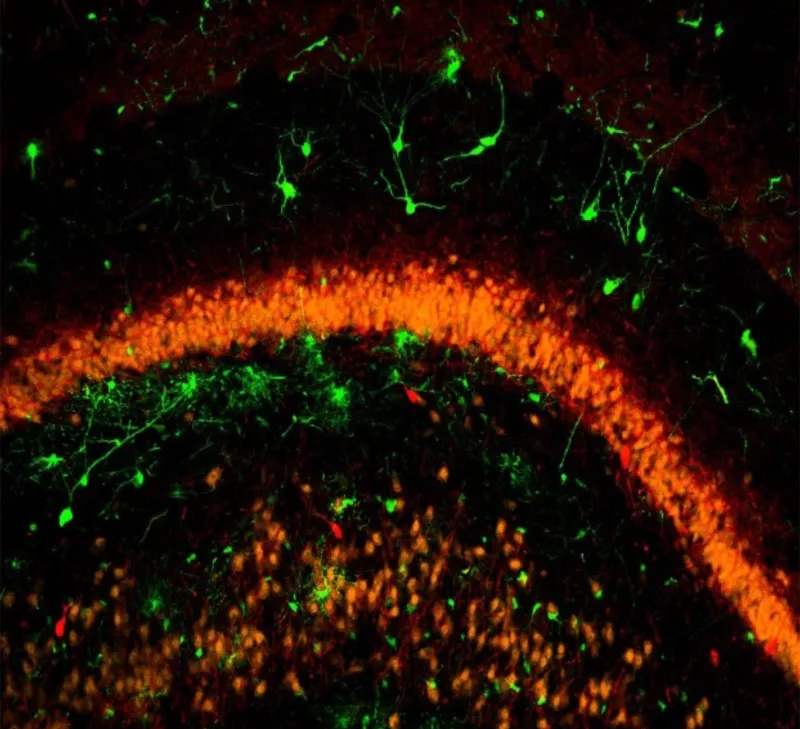
Scientists believe seizures arise from an imbalance between excitatory and inhibitory electrical activity in the brain. Antiepileptic drugs like clonazepam work to treat seizures by increasing the effect of an inhibitory neurotransmitter, GABA. Baraban and his colleagues are instead looking to enhance or restore inhibitory neurotransmission by transplanting precursor GABA-producing inhibitory neurons, or interneurons, into the brain.
In collaboration with UCSF neurosurgery professor Arturo Alvarez Buyulla, PhD, the researchers previously showed that, unlike human stem cells, precursor neurons from embryonic mice can migrate widely when grafted in the brains of adult mice. These transplanted cells can then integrate into the existing neuronal networks in the host animal’s brain.
Over the past two decades, the Baraban lab here at UCSF has consistently demonstrated these transplanted neurons can cure mice of their spontaneous seizures. Interestingly, mice receiving transplanted cells also had less anxiety, a common epilepsy comorbidity.
Baraban, who is also a professor in the Department of Neurological Surgery, says that the success of this interneuron-based cell transplantation therapy relies on the precursor neurons’ unique ability to differentiate into the right kind of interneurons.
While neurons derived from human stem cells do not generate the “right kind” of interneurons, transplanted cells from a region of the embryonic brain called the medial ganglionic eminence generate two key types of inhibitory neurons necessary to enhance inhibitory neurotransmission. An earlier publication from the Baraban lab even suggests that transplanting the “wrong type” of precursor GABA neurons may make seizures worse.
As the scientists work towards translating this therapy from bench-to-bedside, they are now looking to pigs as a source for the embryonic precursor neurons for the transplantations. Their latest study in collaboration with Mercedes Paredes, MD, PhD, an associate professor in the Department of Neurology, indicates that precursor neurons from fetal pigs transplanted into the brains of adult rats migrate and differentiate just like the mouse precursor cells.
“It is important to further study the functional integration of pig precursor cells following transplantation and complete a thorough safety assessment of transplanted cells in a non-human primate,” Baraban said.

He and his colleagues are also investigating how the transplanted interneurons could help treat the other conditions – like anxiety, depression and autism – that often occur with epilepsy.
This interneuron-based cell transplantation using pig precursor neurons has already cured one patient’s epilepsy: Cronutt, a sea lion suffering from seizures caused by domoic acid poisoning from algal blooms along the Northern California coast. Nearly two years after his transplantation, Cronutt remains seizure-free and is even back performing in the sea lion shows at his home in Six Flags Discovery Kingdom in Vallejo.
And that gives the scientists great optimism that their innovative approach, several decades in the making, will soon be ready for intractable epilepsy patients.
