Noncoding RNA genome may promote neural stem cell development, UCSF study says
Even after sequencing the entire human genome, scientists are not yet sure which genes provide the cues necessary to make the many different types of cells during brain development.
Researchers at UC San Francisco have now found that genes that encode proteins and long noncoding RNAs (lncRNAs) each have specialized roles in the earliest stages of neurodevelopment. Their study, published this week in Cell Genomics, provides the most comprehensive view of the distinctions between the coding and noncoding genome during this process.
The results suggest that the lncRNA genome may be responsible keeping stem cells on their specified developmental trajectory. Knowing when and which of these genes to manipulate could help scientists develop cell therapies for treating Parkinson’s disease or stroke recovery.
“This is an important step towards understanding the role of lncRNAs in brain development,” said senior author Daniel Lim, MD, PhD, a professor in the Department of Neurological Surgery.

The researchers used a method called CRISPR interference to “switch off” nearly 30,000 genes in stem cells – spanning the entire coding genome and more than 10,000 lncRNAs. Then, they made 2.7 billion stem cells differentiate into neural stem cells, sorting the cells into those that had differentiated and those that had not based on the presence of a protein marker of early neural development.
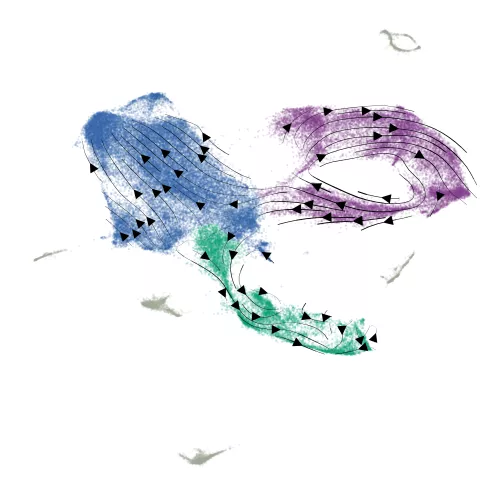
This approach allowed the scientists to compare genes across the coding and noncoding genome. Lim’s research team computed a score for the impact each gene had on stem cell differentiation as well as cell growth. They found that lncRNA genes were far more specialized for roles in differentiation.
“I was surprised to see how much developmental information is encoded in the lncRNA genome,” Lim said.
Previous studies indicated that certain lncRNAs are abundant in the brain, but scientists didn’t have much data on what this group of molecules might be doing in cells. “There was this hypothesis that perhaps most of this noncoding genome is actually noise and not true genes that are actually serving a function,” said David Wu, PhD, the study’s first author and a UCSF MD-PhD candidate. Wu says that these findings may also start to shed light on why lncRNAs, which seem to strongly correlate with the size of an organism’s brain, appeared more recently in evolution.
Carefully “panning” the genetic information from both the coding and noncoding genome with machine learning algorithms, Lim says, helped the researchers prioritize a shortlist of candidate genes to further investigate.
When they combined CRISPR interference with measurements of the abundance of all the genes in single stem cells, the scientists showed “switching off” coding genes made the stem cells get stuck in the process of neurodevelopment.
But “switching off” lncRNAs made the stem cells much more likely to go off into multiple cellular states. The stem cells could even develop into cells far outside the brain lineage, Lim says.

Researchers often rely on gene expression patterns to identify important genes for further study. However, according to Wu, “gene expression patterns were not very predictive of which genes were functional.” To this end, Lim and his colleagues have created a free online resource that allows other researchers to identify coding and noncoding genes that regulate early neural development.
Lim is now interested in examining some of the lncRNAs they identified from their genome-wide screens in human brain organoids – a model better suited for probing the later stages of neural development.
Their observations suggest the lncRNA genome could affect how neural stem cells produce neurons and glial cells or how neurons migrate to the right location in the brain. Lim says their systematic screening approach could be easily adapted to figure out which genes – both coding and noncoding – regulate other biological processes, such as producing other cell types from stem cells. More generally, their approach can even be used to systematically identify new therapeutic targets for human diseases, including brain cancers.
"Detailed studies will be needed to understand how all of these genes control human brain development,” Wu said. “We're excited to see the community use our resource to discover even more about precisely guiding stem cells for regenerative medicine."
